The NanoMILE project intends to establish a fundamental understanding of the mechanisms of nanomaterial interactions with living systems and the environment, across the entire life cycle of nanomaterials and in a wide range of target species. The project will identify critical properties (physico-chemical descriptors) that confer the ability to induce harm in biological systems. This is key to allowing these features to be considered in nanomaterial production (“safety by design”).
The overarching objective of NanoMILE is thus to formulate an intelligent and powerful paradigm for the mode(s) of interaction between manufactured Nanomaterials (MNMs) and organisms or the environment to allow the development of a single framework for the classification of nanomaterial safety and the creation of a universally applicable framework for nanosafety.
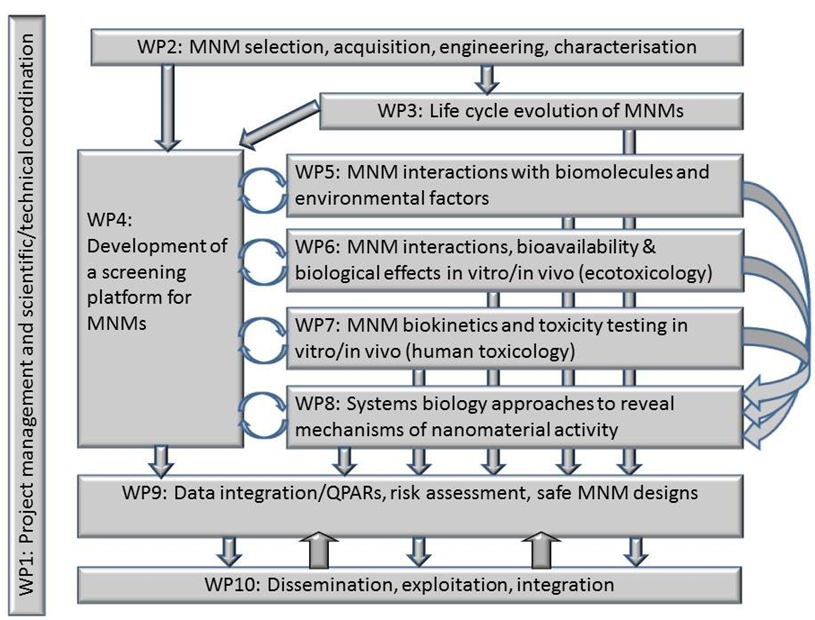
A wide range of manufactured MNMs will be sourced and characterized throughout their life cycle. Using a high throughput screening process, a streamlined testing and selection platform will be developed and applied to refine the MNMs selection. The selected MNMs will undergo focused testing relative to their mechanism(s) of effects on living systems and the environment. An iterative experimental / modeling process will integrate the data obtained into quantitative structure or properties / effects relationships.
The most important outcomes of NanoMILE are expected to be:
- A set of documented protocols for nanomaterials synthesis, characterization & safety assessment, feeding into ongoing standardization activities and building on the work of previous projects;
- MNMs libraries gathering data on structure and transformation in contact with living systems and their connection to toxicity, ecotoxicity, and fate and behavior;
- Mechanistic and quantitative (QSAR/QPAR) descriptions of MNMs properties, and of effects of life-cycle MNMs modifications (aging, interactions with the environment);
- A source for MNMs risk-assessment (dose-response relationships for various dose metrics, target body tissues, biomarkers, biodistribution/ biopersistence);
- A framework for MNMs classification according to their biological or environmental impacts;
- A handbook of best practice (in coordination with the NanoSafety cluster).
NanoMILE will contribute significantly to ongoing efforts to reduce the many uncertainties regarding the potential impact of MNMs on health and the environment, which is urgently needed for the development of a sound regulatory framework. It is crucial to learn which parameters govern the toxicity of nano-sized objects and what the underlying mechanisms are, to support the sustainable development of nanotechnology.
For more information:
- Leaflet of the project (2 pages).
- Description of NanoMILE in the "Compendium of Projects in the European NanoSafety Cluster".